Most effective treatment yet.
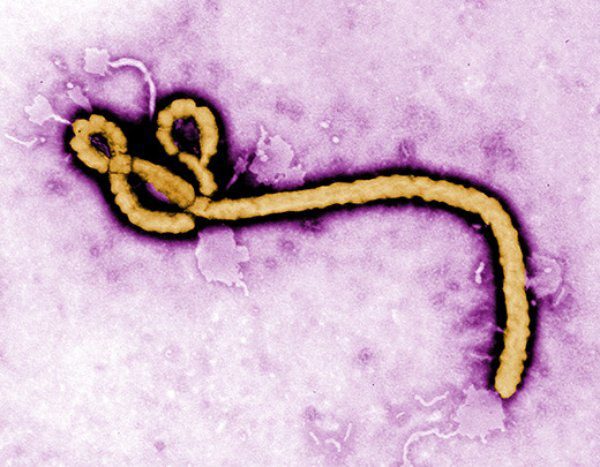
Ebola has been pretty high up there on the list of deadly diseases. After contracting it, assuming you waited too long to get treatment, you may as well flip a coin to see if you’ll survive. Or, at least, that was the case.
Scientists and doctors in the Democratic Republic of Congo have been hard at work on drug trials in a bid to deal with the outbreak of the disease in the region. And on Monday, the World Health Organization and National Institutes of Health announced that two of these experimental treatments have been found to significantly reduce the mortality rates of Ebola.
From now on, we will no longer say that Ebola is incurable.
director general of the Institut National de Recherche Biomedicale, Jean-Jacques Muyembe
There is a vaccine that exists to shield those without Ebola from getting it, but this is the first time a treatment for the disease was ruled to be genuinely effective. While there was a drug (ZMapp) that was previously the standard treatment for the disease, it still had a fairly high mortality rate. Hence the studies in the hopes of lowering the mortality rate.
Patients at treatment centers in the eastern part of the country were randomly assigned one of four treatments. One of them was an antiviral drug called remdesivir, while the others were drug cocktails containing monoclonal antibodies, of which ZMapp was one as a control. Monoclonal antibodies are proteins crafted to recognize the shapes of certain viruses and bacteria, and rally the immune cells to fight them off. The scientists planned to do 725 tests on the new drugs, but stopped at 681; the preliminary results were just that strong.
Per director of the NIH’s National Institute of Allergy and Infectious Diseases Anthony Fauci, ZMapp test cases at the treatment centers experienced a 49% mortality rate; coincidentally, the mortality rate without any treatment is over 75%. The two new monoclonal antibodies, however, preformed spectacularly. The one produced by Regeneron Pharmaceuticals dropped the mortality rate of those fully in the grip of Ebola down to 29%, while NIAID’s mAb114 lowered rates to 34%. Both drugs also did an unprecedented job with patients that had just begun to present symptoms, lowering their mortality rates to 11% for mAb114, and 6% with Regeneron’s drug. By comparison for the same group of early treatment seekers, ZMapp stood at 24% and remdesivir at 33%.
That said, while monoclonal antibodies are useful for the treatment of a large number of diseases, they’re notoriously hard to develop. They often require years of work to create. In the case of ZMapp, that meant many long years of mice with Ebola, and then engineering the antibodies from those mice into something the human body wouldn’t reject. Ebola’s particularly hard to stop, because it’s fairly large and can change shape, so monoclonal antibody cocktails are the go to. mAb114, on the other hand, is the result of harvesting antibodies from human survivors of the disease; specifically from one survivor of the 1995 outbreak in Kikwit, DRC.
The next trial has already been planned, pitting Regeneron’s drug against mAb114. All of the treatment centers in the zone will administer the two most effective drugs, according to WHO’s director of health emergencies, Mike Ryan.
Today’s news puts us one more step to saving more lives. The success is clear. But there’s also a tragedy linked to the success. The tragedy is that not enough people are being treated. We are still seeing too many people staying away from treatment centers, people not being found in time to benefit from these therapies.
Source: Wired